About us
Leading the Next Generation Orthopedic Care
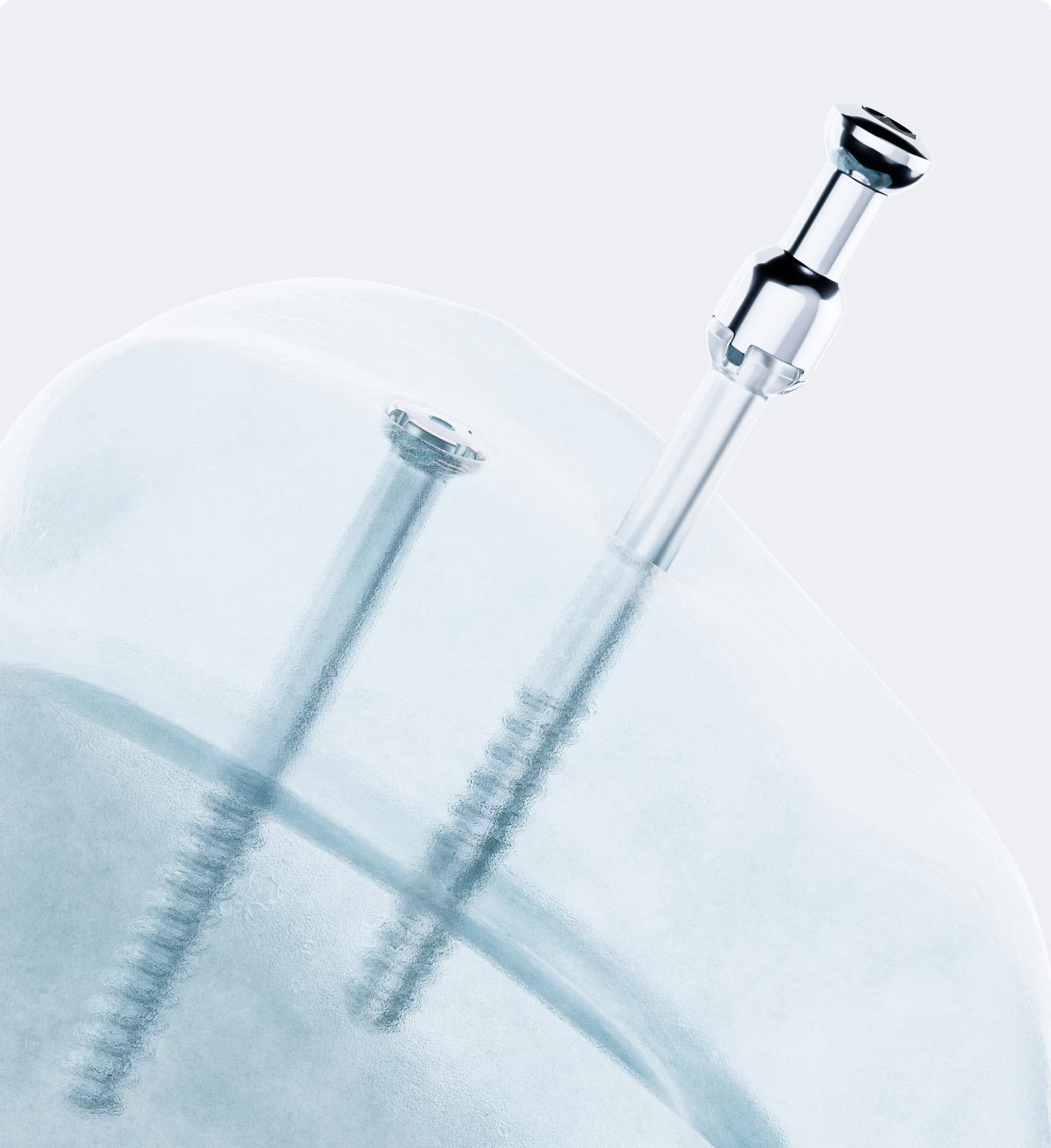

Bioretec is a global pioneer in the design and manufacturing of bioabsorbable orthopedic implants. Founded in 2003 in Finland, the company combines unique expertise in material technology and bioscience to offer superior, yet practical, innovative solutions for fracture treatment that address unmet clinical needs. Today, Bioretec’s products are used worldwide.



Our Mission and Vision
By forging biology and technology, Bioretec’s mission is to unlock the potential of the human body to heal itself and return patients to their unique everyday lives with renewed strength and mobility.
Our vision is a purer world where technology and biology merge naturally to make healing solutions safer, more reliable, and universally accessible.
Our Values

Quality
Bioretec is committed to delivering high-quality, safe products and services that meet the latest industry standards and regulations, ensuring reliability and trust in every solution we provide.

Humanity
Improving quality of life is at the core of everything we do. Our operations are driven by values of integrity and responsibility, ensuring that our actions consistently uphold the highest ethical and moral standards.

Innovation
As pioneers in innovation, we are committed to exploring new technologies and solutions that address unmet clinical needs. Our entire team is united in advancing progress and working toward our shared vision of achieving improved healthcare outcomes for all.
Our Mission and Vision
“Legacy and the Future of the Pioneering Process: We Own Both” encapsulates Bioretec’s journey from its inception to its present-day innovation. This dual commitment to honoring its legacy while steering the future of orthopedic solutions illustrates Bioretec’s unique position in the field.
Founded in 2003 in Finland by Professor Pertti Törmälä, Bioretec initially concentrated on creating next-generation biopolymer implants with controlled degradation rates, which evolved into the Activa product line. Over the years, Bioretec transitioned its focus, leading to a strategic renewal between 2017 and 2020 aimed at addressing the evolving needs of the orthopedic industry. This period reignited product development, emphasizing the innovation of new technologies. This innovative drive culminated in the creation of RemeOs™, a revolutionary implant with no direct market equivalents prior to its introduction. RemeOs has since successfully obtained approval in 2023 for the U.S. market as the first absorbable metal orthopedic implant, marking a significant milestone in Bioretec’s journey.
The transformation of Bioretec into a pioneer of innovation in orthopedic care highlights its dedication to advancing technology and enhancing patient outcomes. With the development of next-generation implants like RemeOs, Bioretec is shaping the future of orthopedic treatment, paving the way for more effective and patient-friendly solutions.
Quality and certificates
We hold an EN ISO 13485:2016 certification and comply with FDA Part 820 requirements, reflecting our commitment to quality and regulatory standards. Most of our products are CE-marked and/or have received market authorization in the U.S. and various other countries worldwide.
The following MDD CE-certificates, granted to Bioretec by DEKRA Certification B.V., formally expired on February 1, 2024. However, their validity has been automatically extended under Regulation (EU) 2023/607, amending Regulation (EU) 2017/745 (MDR), provided the conditions outlined in Article 120(3c) MDR are met:
- Bioretec EC Certificate and Addendum: 2094913CE01
- Bioretec ActivaPin™ PG, ActivaScrew™ PG, and ActivaScrew™ Interference DE Certificate: 2094913DE01
- Bioretec ActivaScrew™ Interference TCP DE Certificate: 2094913DE06
- Bioretec Activa IM-Nail™ DE Certificate: 2094913DE05
This extension ensures uninterrupted compliance and availability of our products in line with regulatory frameworks.
DEKRA Certification B.V., has granted the following MDR CE-certificates:
- Bioretec MDR CE certificate 2250004CE01
- Bioretec RemeOs Screw TE certificate 2250004TD01
Management Team
Board of Directors
Scientific Advisory Board
Dr. Christopher W. DiGiovanni
Member of the SAB since 2025. Professor of Orthopaedic Surgery at Harvard Medical School, Department of Orthopaedic Surgery, and Chief Emeritus of Foot and Ankle Services at Massachusetts General Hospital (MGH) / Newton-Wellesley Foot & Ankle Center, USA. Past President of the American Orthopaedic Foot and Ankle Society and the North American Orthopaedic Foot Club, and co-founder of the MGH Foot & Ankle Research and Innovation Lab (FARIL). He specializes in foot and ankle surgery, with 250+ published articles and numerous contributions to FDA-approved surgical technologies.
Prof. Dr. Jeffrey Wang
Member of the SAB since 2023. Professor of Orthopedic Surgery and Neurosurgery, Co-Director of the USC Spine Center, University of Southern California, Los Angeles, USA. Numerous leadership roles, including AO Spine, the North American Spine Society NASS, the Cervical Spine Research Society, the American Academy of Orthopedic Surgeons. Specializes in spine surgery.
Prof. Dr. Theddy Slongo
Member of the SAB since 2023. Head of Pediatric Surgery and Child Traumatology, University Hospital of Bern, Switzerland. Member and founder of AO Foundation’s Pediatric Expert Group and Chairman of the External Fixator expert group of AO Foundation. Specializing in pediatric orthopedic trauma.
Prof. Dr. Stefan Rammelt
Member of the SAB since 2023. Professor and Head of the Foot & Ankle Section, University Center of Orthopaedics & Traumatology, University Hospital Carl Gustav Carus, Dresden, Germany. President, German Society of Biomaterials. Vice-President, German Foot & Ankle Society. Specializing in foot and ankle surgery.
Prof. Dr. Fan Liu
Member of the SAB since 2021. Chief and Professor in the Department of Orthopedic Surgery at the Affiliated Hospital to Nantong University in China. Prof. Dr. Vice President of the Chinese Orthopedic Association and the Chinese Association of Orthopedic Surgeons. He specializes in orthopedic trauma surgery.
Dr. Robert Leland
Member of the SAB since 2023. Clinical Assistant Professor in the Department of Orthopedics at the University of Colorado, USA. Member of the Colorado Medical Society, American Orthopaedic Foot and Ankle Society, American Academy of Orthopaedic Surgeons, and AO Alumni. Specializing in foot and ankle surgery.
Prof. Dr. Med. Klaus Dresing
Chairman of the SAB since 2021. Former Vice Director of the Department of Trauma Surgery, Orthopedics, and Plastic Surgery at Göttingen University Medical Center in Germany. Numerous earlier leadership positions in AO Foundation. He specializes in orthopedic trauma surgery.
Prof. Dr. Richard Assaker
Member of the SAB since 2023. Professor in Neurosurgery, Hôpital Roger Salengro, Lille, France. Member of the French Society of Neurosurgery, the French Society of Spine Surgery, the Spine Societies of Europe SSE, the North American Spine Societies NASS, he International Educational Committee of the NASS, Chairman of the International education residents’ program. Specializes in spine surgery.
Dr. Verena Schreiber
Dr. Verena M. Schreiber, MD, FAAOS - Member of the SAB since 2023 Associate Professor, Department of Orthopaedic Surgery, Cincinnati Children’s Hospital Medical Center, USA. Member of the Pediatric Orthopaedic Society of North America, Orthopaedic Research Society, Orthopaedic Trauma Association, American Academy of Orthopaedic Surgeons, and American Academy for Cerebral Palsy and Developmental Medicine. Specializes in pediatric orthopedic trauma.
Work With Us
With dedication and care, we’ve worked ambitiously to create something we believe will transform the industry and improve lives. Want to work with us?